Introduction CAR-T efficacy in AML has been limited by a lack of ideal target antigens as well as the aggressive and resistant nature of disease. CD123 expression on leukemic blasts has been observed in about 80% of AML patients. However, the antigen is also expressed on non-malignant hematopoietic cells including neutrophils and endothelial cells. As consequence, targeting of CD123 with conventional CAR-T has been shown to result in persistent cytopenias, capillary leakage syndrome, CRS, and ICANS. AVC-101 (UniCAR02-T-CD123) is an adapter CAR-T, consisting of a universal CAR-T cell (UniCAR-T) and a CD123 targeting module (TM). The TM binds to the CAR-T cell via a peptide tag and induces T cell activity against CD123 bearing cells. Due to the short half-life of the TM, interruption of its continuous IV administration will rapidly switch T cell activity off, and mitigate toxicities as shown previously. Since the presentation of initial results, the study was amended to include a stricter and prolonged schedule of TM consolidation cycles. Given the excellent safety enabled by the switchable mechanism, additional dose levels (DL) of TM were added.
Methods Key inclusion criteria included rrAML patients with ECOG ≤ 1, CD123 expression in ≥ 20% of blasts, and no suitable approved treatment option available or MRD positive AML patients. Initial dosing was applied using an adaptive Bayesian optimal interval design for drug-combination trials, escalating either cell dose or TM concentration based on posterior probability calculation of AEs. Dose escalation for the two additional dose levels was guided by a classical 3+3 design. Patients received standard Flu/Cy lymphodepletion (LD) for 3 days and TM was administered as continuous infusion, whereas UniCAR-T cells were given as one-time infusion on cycle 1 day 1. After completion of the initial 20-day induction cycle, responding patients were allowed to receive up to 3 consolidation cycles of 12 days continuous TM infusion with 7-14 days treatment free intervals in between cycles.
Results As of this time 19 patients have received the induction cycle, 8 patients have also received at least one consolidation cycle (1-3, total 13 cycles). Patients were heavily pretreated with a median of 4 (2-7) prior treatment lines. Twelve of these patients had received a prior allogeneic (Allo) stem cell transplantation (SCT). Most of the patients (60%) were in adverse risk category according to ELN classification. In the initial escalation phase, 19 patients were treated in 16 dose cohorts. We observed a single DLT at DL 8 (reversible drop in fibrinogen levels, reported earlier) but no DLTs occurred in 11 patients treated in the yet highest dose cohort of 4 mg/day TM and 500 million UniCAR-T. Treatment was generally well tolerated irrespective of age and prior treatments. CRS was observed in 12 patients, mostly grade 1 or 2. Three grade 3 CRS and 1 grade 2 ICANS were reported, all resolving within 24 hrs from interruption of TM administration. Treatment-related AEs were most frequent in cycle 1, none were reported in cycles 3 and 4. No treatment-induced lasting myelosuppression was observed, and no patient required SCT support for WBC reconstitution.
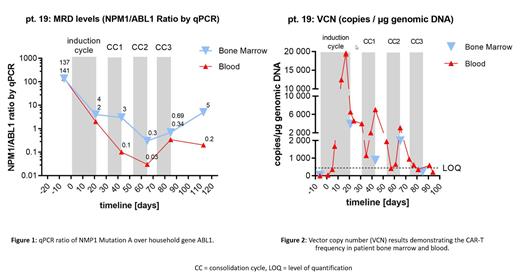
ORR in the first 19 patients was 53% (8/15) for the rrAML population and 75% (3/4) for the MRD population. Since the last presentation, extended administration of TM (20-day induction followed by three 12-day consolidation cycles 1-2 weeks apart) initiated by protocol amendment resulted in durable, ongoing responses approaching 5 months, longer than any prior. A 64-year-old MRD+ patient (#19) with 4 prior lines of therapy experienced a 3-log reduction in MRD levels (NPM1 mutation by qPCR) in both bone marrow and blood (Figure 1) and became transplant eligible. Re-administration of TM resulted in a significant re-expansion of UniCAR-T (Figure 2) without further cell dosing or LD. Another patient (#20) with overt hematological relapse after AlloSCT achieved a CR, ongoing at 5 months.
Full safety and efficacy results of the additional 2 dose cohorts with prolonged TM consolidation will be presented at the meeting.
Conclusions AVC-101 continues to be safe and tolerable. Repeated dosing of TM in consolidation cycles resulted in robust re-expansion of UniCAR-T with deeper remissions of extended durability. The switchable mechanism provides proven rapid reversal of safety events, enabling higher dose levels.
Disclosures
Wermke:Immatics: Consultancy; Lilly: Consultancy, Honoraria; MSD: Consultancy; Boehringer Ingelheim: Consultancy; Pfizer: Consultancy, Honoraria, Other: Travel, accommodations, expenses; Cellex GmbH: Consultancy; Novartis: Consultancy, Honoraria; GeMoab: Consultancy, Other: Travel, accommodations, expenses; AstraZeneca: Consultancy, Other: Travel, accommodations, expenses; ISA Pharmaceuticals: Consultancy; Amgen: Consultancy, Other: Travel, accommodations, expenses; Roche: Consultancy, Honoraria, Other: Travel, accommodations, expenses, Research Funding; Bristol-Myers Squibb: Consultancy, Other: Travel, accommodations, expenses. Kraus:Jazz Pharmaceuticals: Honoraria, Other: Support for meeting attendance; BMS: Honoraria, Other: Support for meeting attendance; Gilead: Other: Support for meeting attendance; LinkCare: Honoraria; AstraZeneca: Speakers Bureau; Janssen: Honoraria; Pfizer: Honoraria. Sala:Novartis: Honoraria; Takeda: Consultancy; Medac: Other; Kite Gilead: Consultancy, Honoraria, Other: Support for meeting attendance; BMS: Consultancy, Honoraria, Other; Jazz Pharmaceuticals: Consultancy, Honoraria, Other: Support for meeting attendance. Vucinic:Janssen: Honoraria; Gilead/Kite: Consultancy, Honoraria; Amgen: Honoraria; MSD: Consultancy, Honoraria; Sobi: Honoraria, Other: Travel/Accommodations/Expenses; Takeda: Consultancy, Honoraria; AstraZeneca: Honoraria; BMS/Celgene: Consultancy, Honoraria, Other: Travel/Accommodations/Expenses; Novartis: Consultancy, Honoraria; Abbvie: Honoraria. Fiedler:AbbVie: Consultancy, Honoraria, Other: Support in medical writing; Morphosis: Consultancy; Apis: Research Funding; Jazz Pharmaceuticals: Consultancy, Other: Support for meeting attendance; Servier: Consultancy, Other: Support for meeting attendance; Pfizer: Consultancy; Stemline: Consultancy; Clinigen: Consultancy; Amgen: Consultancy, Other: Support for meeting attendance, Patents & Royalties. Goebeler:Janssen-Cilag: Consultancy, Honoraria; Bristol-Myers Squibb (BMS): Honoraria; Amgen: Consultancy; AstraZeneca: Honoraria; Novartis: Honoraria; Gemoab: Consultancy; Roche: Honoraria. Koedam:AvenCell: Current Employment, Current equity holder in private company. Franke:AvenCell Europe GmbH: Current Employment. Vänskä:AvenCell Europe GmbH: Current Employment; ICON plc: Ended employment in the past 24 months. Ehninger:AvenCell Europe GmbH: Current Employment; AvenCell Therapeutics, Inc.: Current equity holder in private company, Current holder of stock options in a privately-held company. Cartellieri:AvenCell Europe GmbH: Current Employment; AvenCell Therapeutics, Inc.: Current holder of stock options in a privately-held company. Ehninger:AvenCell Europe GmbH: Current equity holder in private company, Ended employment in the past 24 months, Membership on an entity's Board of Directors or advisory committees, Other: Support for meeting attendance; CelleCell Professionals GmbHx: Current Employment, Current equity holder in private company. Maniar:AvenCell Therapeutics, Inc.: Current Employment.